| Home | Research | Lab Members | Publications | Collaborations | Support | Upcoming Events | Contact Us |
We developed a new strategy to detect BrdU in whole mounts of seminiferous tubules by immunofluorescence. The immunopositive cells can be seen as chains or cohorts of spermatogonia or preleptotene spermatocytes. Our data reveal that premeiotic germ cell expansion follows a clonal scheme.
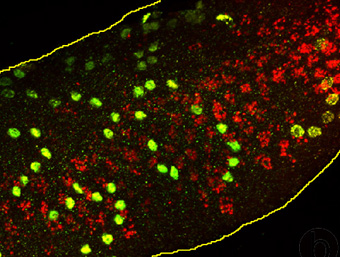
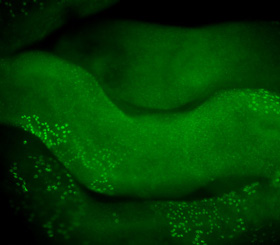
When the BrdU localization (green) is combined with determination of acrosome development (acrosin staining, red) and confocal imaging, we are able to describe a stage-specific appearance of S-phase in rhesus monkey testes and to determine number and size of clones at each spermatogenic stage. We determined that spermatogenesis starts at stage VII with a first division of pairs or quadruplets of Ap spermatogonia. Clonal splitting after this initial division is followed by a second mitotic step after which no splitting occurs. Cells now arrest generating new Ap pairs or quadruplets or generate clonally expanding chains of B-spermatogonia.
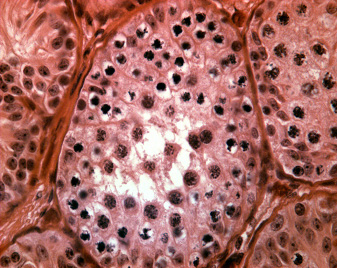
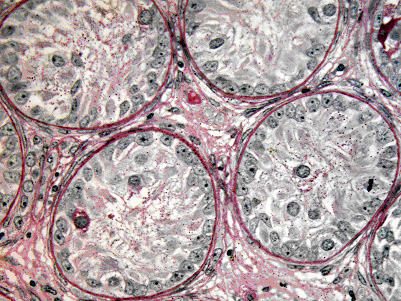
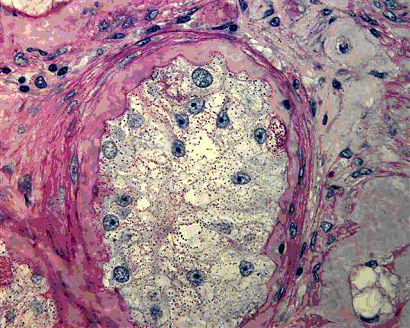
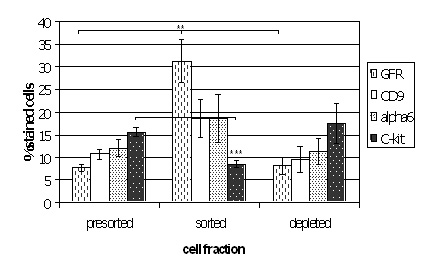
Magnetic activated cell sorting was applied to enrich spermatogonial stem cells from immature mouse testes. After isolation the cells were stained immunohistochemically for additional spermatogonial markers. The plot shows that the sorted fraction was significantly enriched for GFRA-1 when compared to presorted and depleted fractions. In addition, a significant depletion of c-kit positive cells was encountered. These data show that the technique can be applied to obtain enriched fractions of spermatogonial stem cells.
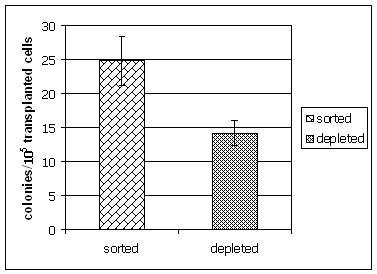
The enrichment of spermatogonial stem cells was confirmed by germ cell transplantation experiments performed in collaboration with Kyle Orwig, Pittsburgh Development Center, Magee Woman’s Research Institute. Mouse testes receiving sorted cell fractions generated significantly more spermatogenic colonies in busulfan depleted recipients when compared to mice receiving cells from the depleted fraction.
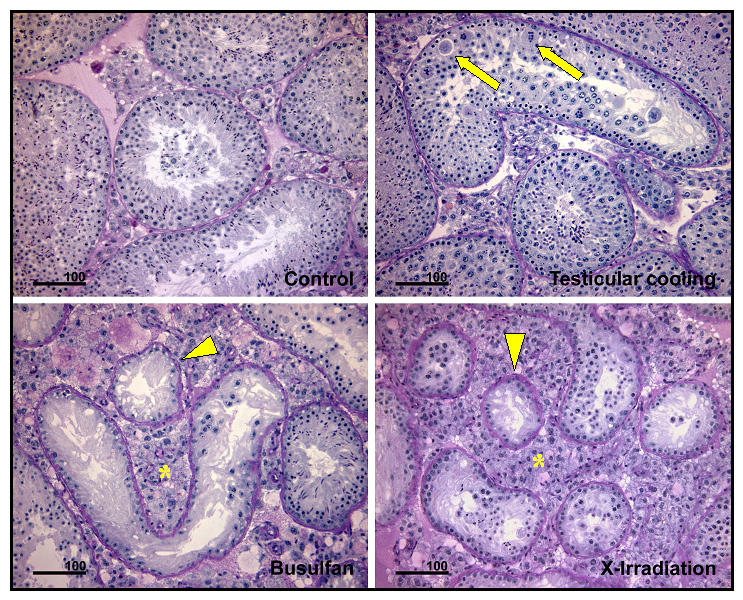
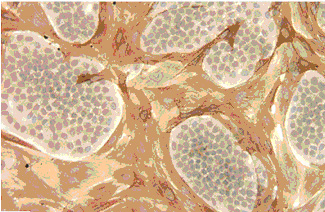
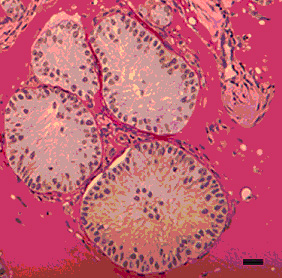
We explore the effects of aging on testicular stem cells in mouse and monkey models. This plate of micrographs shows the effect of cooling, busulfan and X-irradiation on the testis of old Balb-c mice. In comparison to control testes, minor disorganization is observed after cooling. In contrast, busulfan and X-irradiation show a severe depletion of spermatogenic cells. This study revealed that the degree of damage following these exposures are similar in old and young mice.
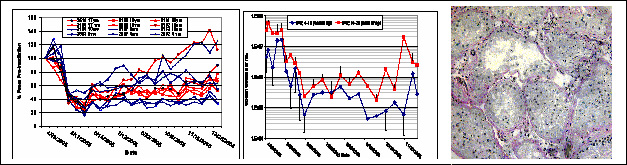
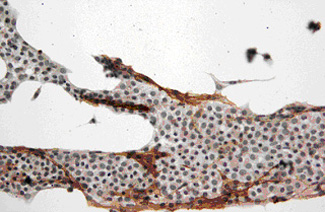
The two plots show the effect of testicular irradiation (4Gy) on individual testicular volume (left panel) and sperm counts (middle panel) in groups of young adult (red) and aging (blue) rhesus monkeys. Radiation exposure evoked an almost complete depletion of germ cells as becomes evident from a representative testicular biopsy (Mk3058) obtained 8 weeks after irradiation shown as micrograph in the right panel.